AXSED conforms toYY/T0664 Medical Device SDLC process with our professional software-development team collaborating with the lawyer team, filing the design and development, doing our bit to help develop the registration of medical software for companies.
AXSED provides clients with professional, compliant solutions for software development, most suitable development model, professional software testing and strict RM(risk management) to satisfy every need for a perfect software.

AXSED shortens the period of compliance development, and reduces budgets of R&D and risks for companies.
Filing Development.
Demand Analyzing.
Elaborate Design.
Unit Testing.
Integration Testing.
System Testing.
Risk Management.

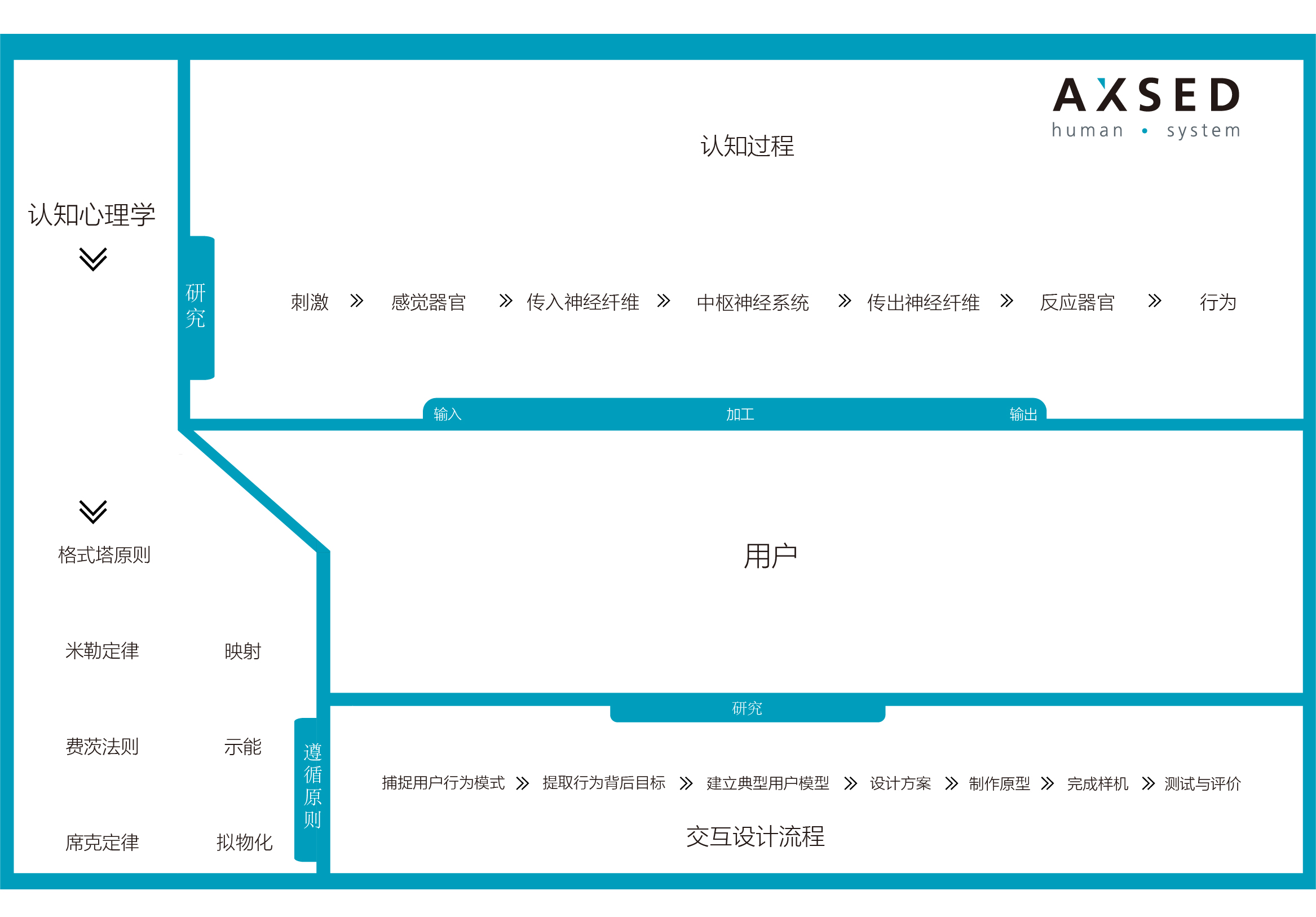
In accompany with the provision of compliant (YY1474/IEC62366,YY0316/ ISO14971) development of software, AXSED is also proficient in the agile and compliant(ISO9241, YY0664/IEC62034)implementation of usability of User Interface (UI). This conforms to the intuitive operation of cognitive psychology, and meets the requirements for safety and effectiveness of medical software to most extent through better avoiding risks caused by operation, which guarantees good user experience and brand loyalty.